October 20, 2015
Untruths We Were Told about Ebola
Notice: Undefined index: gated in /opt/bitnami/apps/wordpress/htdocs/wp-content/themes/thenewatlantis/template-parts/cards/25wide.php on line 27
A warning we have yet to heed
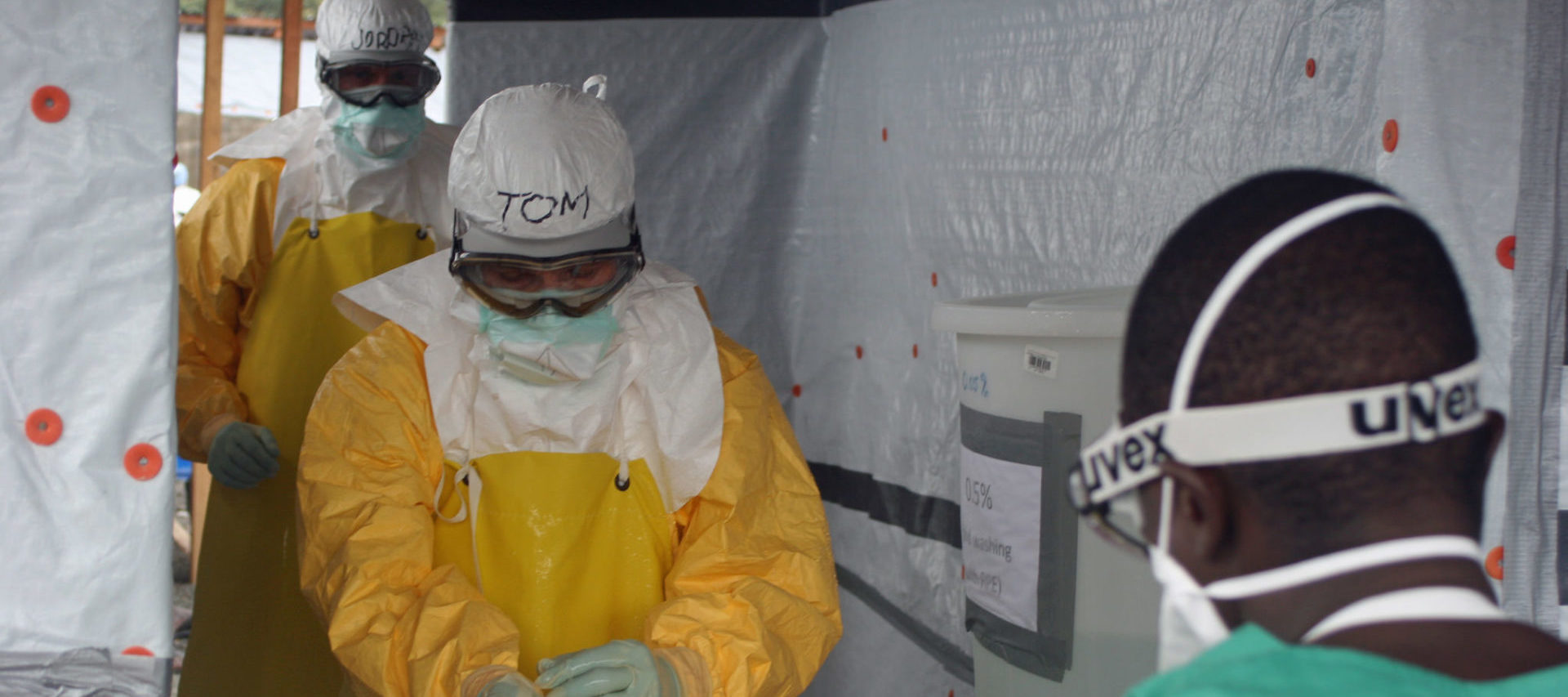
There was a peculiar moment during a news segment last fall, at the height of the Ebola scare in the United States, between the nation’s two most recognizable medical figures. Sanjay Gupta, CNN’s household-name medical correspondent, was standing next to Thomas Frieden, the director of the Centers for Disease Control and Prevention, in front of CDC headquarters in Atlanta on October 1. Frieden assures Gupta that if one of them had Ebola, the other would be at no risk of infection: “It’s not like the flu, not like the common cold. It requires direct physical contact.” A CNN anchor interjects: “But if he sneezes on you, it’s a different story.”
Gupta laughs nervously and Frieden shrugs. Gupta then asks whether their close proximity would not in itself qualify as a contact under the guidelines the CDC uses to trace the spread of outbreaks. Frieden does not clearly answer the question, but reiterates that during the conversation the two have had no direct contact between their bodily fluids, implying that that would be the only way their proximity would pose a risk. Smiling playfully, Frieden seems to suggest that Ebola is an illness of intimates.
Returning to the previous question, Gupta then asks, “The reason we talk about coughing and sneezing not being a concern — if you were to have coughed on me, that — you’re saying that would not be of concern?” Frieden’s answer, in full:
We would look at that situation very closely to see at what point in the person’s illness, and — you know, we’re always gonna err on the side of caution.
The interview ends with the two men shaking hands.
Live television can be a tricky medium, and minor hiccups should be treated forgivingly. Yet this was the top public health authority in the United States, on the morning after he had announced that the first patient in his country had tested positive for Ebola, a possibility that had been discussed for months as the largest outbreak in history ravaged West Africa. There was no task more definitive of the job of CDC director, nor one more foreseeable, than having clear answers to those questions on that day.
And on that day those questions had become of immediate, life-or-death consequence. The assertion that Ebola cannot be transmitted through the air was the basis for the CDC’s recommendation that the only respiratory protection needed for health care workers providing routine care to Ebola patients was surgical masks, to guard from splashes of bodily fluids. And Frieden’s demeanor here — of reassurance tinged with condescension and even evasion — was not a one-time flub. Rather, it was representative of his communication style during the outbreak, and of the broader response by the CDC and public health leadership.
In December 2014 and February 2015, after public attention to Ebola had waned, two medical articles quietly appeared that exhaustively summarized the available literature on how Ebola is transmitted. In the gently descriptive language of scientists, these papers walked through most of the CDC’s and the World Health Organization’s major claims about Ebola — that it cannot be transmitted via water supplies, sewage, or the air; that its maximum incubation period is 21 days; that patients are contagious only when symptomatic — and showed how each, though stated definitively on posters and in public statements by the CDC and the WHO, was not only based on fragmentary evidence, but had evidence to qualify or contradict it.
In perhaps no case was the gap larger or more consequential than on the question of transmission through the air. The second of the two papers — published in the open-access journal mBio and coauthored by 21 infectious-disease researchers from across the world, including many of the most recognized and respected names in the field — concluded, “It is very likely that at least some degree of Ebola virus transmission currently occurs via infectious aerosols.” The research summarized in these papers was almost entirely published before the outbreak of 2014.
In fact, even during the height of the outbreak, several experts warned that there was tentative but extensive evidence that Ebola may sometimes be transmitted through the air. Articles published in September 2014 in the International Journal of Nursing Studies and on the website of the Center for Infectious Disease Research and Policy at the University of Minnesota cautioned that surgical masks likely do not offer adequate protection to health care workers treating patients with Ebola. Drawing on years of research, some of it conducted by the authors themselves, both articles urged the CDC and the WHO to change their recommendations to include the use of respirators — masks that filter contaminants from the air or supply fresh air — and called on them to direct funding to fulfill this need for health care workers in West Africa.
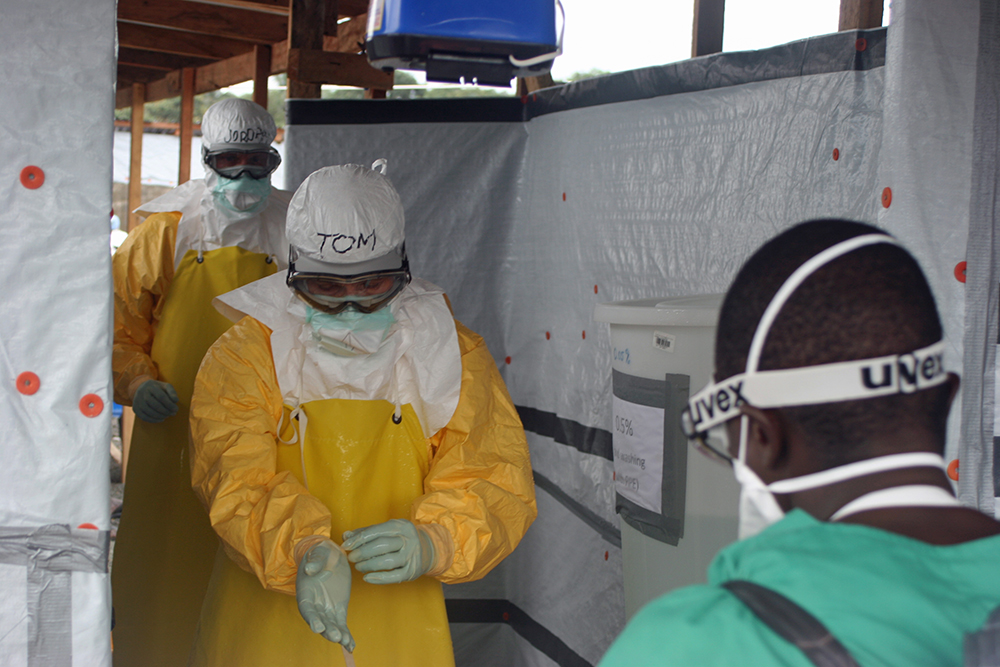
Officials at the CDC and the WHO were aware of these articles, and the research they drew on. Frieden himself, when he visited a Doctors Without Borders treatment center in Liberia in August 2014, had worn that organization’s protections, far more stringent than CDC guidelines: goggles, a full-body impermeable suit designed to leave no inch of skin exposed, and, underneath, a respirator (see above). But both the CDC and the WHO stuck to their position that surgical masks offered adequate protection.
Then, in October 2014, weeks after those articles were published, the facts on the ground changed. On October 10, a nurse in Dallas who had been treating an Ebola patient tested positive for the virus. On October 13, a second of the patient’s nurses, experiencing a low-grade fever, phoned the CDC to request permission to take a domestic flight. The CDC granted it, and the nurse took the flight the same day. On October 15, she tested positive for Ebola. Though a definitive investigation of the infections has yet to be released, the chief clinical officer for the nurses’ employer stated at the time that both nurses were following CDC guidelines, including the use of surgical masks, but not respirators. On October 16, President Obama repeated that Ebola is not airborne, while Dr. Frieden was grilled by a congressional subcommittee about the CDC response. Finally, on October 20, the CDC upgraded its recommendations for Ebola workers to include respirators under all circumstances.
What follows is an investigation into the failures of the CDC and the WHO in protecting health care workers from Ebola virus disease: how the agencies became averse not to risk but to acknowledging risk; how they did not “err on the side of caution” but guarded only against dangers for which the evidence was firmly conclusive; how they became as concerned with reassuring the public as with actually protecting it.
Moreover, the failings of both agencies were not only negligent but knowing. Investigations for this article have uncovered evidence that the CDC and the WHO may have exerted pressure against researchers who were raising alarms that the agencies’ safety guidelines were too lax — and may still be doing so.
But the failures extend beyond these agencies to a broader malaise afflicting medicine and public health. It is partly scientific: The conventional model of how infectious diseases spread, now nearly a century old, is beginning to crumble under the weight of new research. But the malaise is more deeply cultural: Medical and public health professionals have become entrenched in convention, well beyond the point of beneficial conservatism. In many cases they have not only failed to challenge outmoded views and practices, but have mobilized to shame and marginalize critics who dare to question their ways.
The attention of political leaders, the news media, and the general public has largely moved on, even though the threat of emerging infectious diseases has far from passed and the Ebola outbreak itself continues in West Africa. And although a few particular CDC recommendations have changed, the broader institutional factors that led to the failures of public health in 2014 remain unchanged. We must understand and fix these problems, for the next outbreak may be of a disease more contagious than Ebola, and even worse understood.
During the outbreak that began in 2014, public discussions about whether Ebola could be transmitted through the air were largely centered on variations of two questions: (1) Is Ebola airborne, like the flu and measles? (2) If Ebola was not airborne in previous outbreaks, has it evolved to become airborne now?
The answer to both questions is something close to no. And it was almost entirely on these terms that concerns about transmission of Ebola through the air were raised and dismissed by the public health leadership and journalists alike.
But these questions were not enough.
There had been only about 2,400 known cases of individuals ever infected with Ebola prior to the 2014 outbreak. The available epidemiological data showed that the large majority had had direct physical contact with someone else who was ill with the disease. This would seem to suggest, in answer to the first question, that in past outbreaks Ebola spread through direct contact and was not capable of spreading through the air, like the flu is thought to. (We will return to this question in a moment.)
The question of whether Ebola had mutated significantly from previous strains was hotly debated during the outbreak. This was largely on speculative grounds: the outbreak’s unprecedented size — by October 1, 2014 there had already been more than 7,400 cases and 3,400 deaths, figures that have since tripled — suggested that the infectivity of Ebola might have markedly increased. On the other hand, epidemiological studies of the current outbreak continued to point to direct-contact transmission.
Then, in March 2015, a paper in the journal Science described the results of a major effort to sequence Ebola virus genomes. It concluded that the virus “is not undergoing rapid evolution in humans during the current outbreak.” Of course, even small, slow genetic changes can result in large, rapid changes in the characteristics of a virus, including sudden jumps in virulence. For example, a recent study in the journal Nature Communications reports that, in the bacterium that causes bubonic plague, one gene acquisition along with a single mutation in that gene may have caused a sudden jump in virulence that enabled the Black Death. There’s still good reason, then, to study Ebola’s evolution, and some scientists are continuing to do so.
But the consensus explanation for why the disease has spread so quickly this time involves the unique set of vulnerabilities of West African countries: poor health staffing, equipment, and funding; poor training in hospitals in combating epidemics, and in general best practices; lack of public health infrastructure to perform the contact tracing vital to stopping transmission; recent increases in population density and urbanization; cultural norms of extensive casual physical contact between friends and acquaintances; and particularly tragically, the practice of ritual touching of the dead, which accounted for a substantial portion of infections — in one case over three hundred deaths were linked to a single funeral. There is also research suggesting that weather conditions may have been especially favorable to virus transmission. In short, a large outbreak was probably inevitable, and aggravating conditions had failed to converge so fully in prior outbreaks only by chance.
The question of mutation and increased virulence was vital. The trouble was not with the question itself, but that it was often the only one asked in discussions of Ebola transmission through air. The discussion rarely reexamined whether it can do so in its present form.
Understanding the problems with the other question — of whether Ebola can transmit through the air, as the flu is thought to — is more complex. The conventional model of infection breaks disease transmission into four modes relevant to a hospital setting:
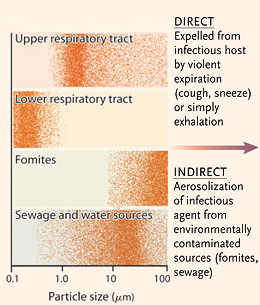
● Direct contact: Requires physical contact with the body of an infected person, usually with bodily fluids exposed through the eyes, mouth, nose, genitals, anus, or open wounds. This mode includes sexual contact, touching the dead, handshakes and casual touching where fluids could be exchanged, and medical procedures that involve contact with the inner respiratory tract, inner parts of the body normally concealed, or other wet tissues.
● Indirect contact: Like direct contact, but fluids are transmitted via some external surface — such as a doorknob or a countertop — where they remain viable outside the body. Indirect contact is usually grouped together with direct contact under the broader category of “contact transmission.”
● Droplet: Involves the expulsion into the air of small particles of bodily fluids, for example through vomiting or expectoration. These are particles that are too heavy to remain suspended in the air, and so can pass from an infected individual to a new host only within a very short distance and time. Droplet transmission is sometimes described as a form of contact transmission.
● Airborne: Involves the transmission of viruses or bacteria, suspended in liquid droplets small enough to float in the air and cause infection over long distances. Airborne particles can be produced by coughing, sneezing, or certain aerosol-generating procedures like intubation.
These modes of transmission are generally regarded as well-defined and distinct. Each is thought to involve a largely separate set of physical mechanisms for the transmission of infectious particles from one person to another. And to some extent, each is thought to involve its own sets of mechanisms for the production of infectious particles by a sick individual, as well as for the absorption of these particles by the tissues of a new host.
Perhaps most significant is that each of these modes of transmission matches up with a corresponding level of precautions. Widely recognized by public health agencies and hospitals, the levels, as defined by the CDC, are:
● Contact precautions (covering both the direct and indirect modes of transmission): Place the patient in an exam room if he or she is visibly leaking bodily fluids. Wear gloves when touching patients or their belongings. Wear a gown if “substantial” contact is expected.
● Droplet precautions: Place the patient in an exam room with a closed door; if this is not available, provide the patient a surgical mask and move him or her as far from other patients as possible. Wear a surgical mask. If “substantial spraying of respiratory fluids is anticipated,” also wear gloves, gown, and goggles or a face shield.
● Airborne precautions: Have the patient enter the hospital through a dedicated isolation entrance, and proceed immediately to a ventilated isolation room. If fluid spraying is expected, also wear gloves, gown, and goggles or a face shield. All personnel in the room with the patient must wear respirators.
These levels are in addition to the standard precautions, which apply in all situations, and include hand hygiene; use of gloves, gowns, and surgical masks, depending on anticipated exposures; cough etiquette; safe injection practices; and so on.
The precaution levels that apply to each disease match up with the disease’s known transmission modes. A set of 2007 guidelines from a CDC advisory committee shows this neat breakdown: smallpox requires airborne and contact precautions; Ebola requires droplet and contact precautions; streptococcal pneumonia requires only droplet precautions; SARS requires all three; anthrax, HIV, and staph require only standard precautions.
These guidelines often establish further subdivisions based on a patient’s stage of illness, the procedural context, and other factors. For example, health care workers near patients with diseases transmitted via droplets may not need to follow droplet precautions unless there is a possibility that they will be within droplet range of the patient, conventionally defined as three feet. Conversely, diseases that are not considered airborne may still require airborne protection when workers will be performing procedures that generate aerosols from bodily fluids (although exactly which procedures do so is not well understood).
Some questions about the ostensibly sharp distinctions between these modes of transmission should be apparent. Coughs and sneezes, for example, would seem to fall under both droplet and indirect contact transmission, and possibly aerosol too. The 2007 guidelines actually list coughing as relevant to both droplet and airborne precautions.
The distinction between airborne and droplet transmission is especially important — and especially unclear. How quickly do the particles have to settle out of the air to qualify as “droplets”? And how big do they have to be? Which bodily events produce airborne particles, which only droplets? Which exposed wet tissues are vulnerable to infection by which type of particles? The answers to these questions are widely regarded as clear-cut, although in fact they vary significantly between sources.
The 2007 guidelines say that the boundary between droplets and smaller particles is generally held as a diameter of 5 micrometers, or millionths of a meter. The range of possible droplet transmission from an infected patient, meanwhile, is generally considered 3 feet. But some guidelines from other sources say 6 feet, while others still state only that droplets cannot infect over long distances, without specifying.
There is more than a whiff of arbitrariness here. As we will see, these divisions have more to do with simplifying assumptions that were made long ago and have only recently been challenged than they do with any distinctions found in the mechanics of disease transmission in the real world.
Much of the public confusion between modes of transmission owes to the terms droplet, airborne, and aerosol themselves. These three words have everyday meanings that are at odds with the meanings attached to them in scientific contexts — meanings that vary significantly between contexts, even though most scientists treat them as precise and settled.
In most publications, the term aerosol encompasses the particles involved in both the airborne and droplet modes of transmission. A topic sheet published in 2010 by the CDC’s National Institute for Occupational Safety and Health (NIOSH) defines an aerosol as “a suspension of tiny particles or droplets in the air.”
But “aerosol” transmission is sometimes used interchangeably with “airborne” transmission. Part of the confusion arises just from the similarity between the words. But it gets even more confusing. The word “droplet” is used as both an adjective and a noun: it refers to both a mode of transmission and the kinds of particles involved in that mode. But the word “airborne” refers only to the mode of transmission. It is just an adjective, and does not refer to the particles involved, for which there is apparently no generally accepted term. In this article, where necessary, I will simply refer to these as “airborne particles.”
Since there is not a conventional short term for airborne particles, when one is needed, the broader term “aerosol” is often used, giving the misimpression that this word refers specifically to airborne particles. The NIOSH topic sheet, for example, mentions a document titled “Generation and Behavior of Airborne Particles (Aerosols).” Adding yet further confusion, sometimes airborne particles are instead described as “droplets” — usually “small droplets.” Further, some occupational safety documents introduce additional terms like “vapors” and “fumes.”
This terminological chaos contributed a great deal to the confusion during the Ebola outbreak, as media outlets focused on the question of whether the virus was “airborne.” Some public health figures then began to assert that Ebola was not airborne by re-labeling droplet transmission as “splashing.” Sometimes they suggested this splashing is just a form of direct contact.
The confusion deepened when, among the CDC and media outlets, the (correct) claim that droplet transmission is not airborne transmission was treated (incorrectly) as refuting that Ebola is airborne, and (even more incorrectly) as refuting that Ebola can be transmitted through the air. Numerous CDC fact sheets offered statements like “Ebola is not spread through the air,” a mistaken rendering of the already poorly founded claim that Ebola is not transmitted through the airborne route.
Perhaps instead of “airborne” and “droplet,” better labels — usefully uncommon in this context — would be mist versus projectile transmission. Because the airborne–droplet model is so pervasive, it is useful to clarify it — even though, as we will see, it would be better to discard it entirely. As an analogy, imagine one of those Mythbusters episodes in which explosives are detonated at a desert firing range, and a great cloud of debris flies into the air. The projectiles are the bits of rock arcing away from the explosion. The mist is the cloud of dust kicked up. The projectiles fall quickly out of the air, only traveling some definite distance, while the mist lingers, floats, and disperses over an indefinite distance. This analogy illustrates how airborne particles (or mists) require the air to disperse, while droplets (or projectiles) do not. Mists travel by aerial buoyancy, projectiles by momentum. If the Mythbusters explosion happened on the Moon, both mist and projectiles would fall quickly to the ground.
The scientific term airborne transmission, then, requires a doggedly literal reading. When public health officials insist that Ebola is “not transmitted through the air” or is “not airborne,” what they mean is that while droplets carrying Ebola may happen to pass through the air, they are not borne by the air. So much depends upon a preposition.
All of which returns us to the question: Can you get Ebola from a sneeze or not? The answer provided by the CDC was ambiguous and ambivalent during the outbreak and remains so now. A little digging through the agency’s fact sheets and public statements shows a series of confusing, contradictory, and misleading statements on this question. And the Internet Archive reveals a flurry of changes to the fact sheets in particular during late October. But the employees of the CDC press office should not be criticized too harshly for this frenzy of revisions. For the distinctions and the models they were tasked with explaining are deeply confusing — and, it turns out, deeply confused.
The distinction between the airborne and droplet modes of transmission arose in the early twentieth century. In his influential 1910 book The Sources and Modes of Infection, Charles V. Chapin, an epidemiologist and public health official, asserted that “there is no evidence that [infection by air] is an appreciable factor in the maintenance of most of our common contagious diseases.” Therefore, we are “warranted … in discarding it as a working hypothesis and devoting our chief attention to the prevention of contact infection.” Droplet transmission was not yet seen as a distinct mode of transmission, but Chapin hinted at it when he wrote of diseases that are “spray-borne only for two or three feet.”
In 1919, George H. Weaver, the physician-in-charge of Chicago’s Durand Hospital for the poor, unequivocally identified droplet transmission as distinct from both aerial and contact transmission, arguing that it “has not usually received sufficient attention.” Previous researchers, Weaver wrote, saw droplet infection as a kind of contact transmission, partly because they failed to consider the distances droplets could travel. Weaver argued that experiences during the recently concluded world war “have served to emphasize the ease with which infections may be transferred through mouth droplets when people are brought into intimate association.” As he wrote, an influenza pandemic was raging that would eventually claim more lives than the war itself.
Later research hardened the distinction between droplet and aerosol transmission. In a 1936 paper in the Journal of the American Medical Association, William and Mildred Wells calculated that liquid droplets smaller than 100 micrometers typically evaporate before reaching the ground, leaving the solid infectious bits to float in the air like particles of smoke, and disperse widely. The Wellses concluded that the two modes of transmission “are by nature opposite. Droplet infection is essentially localized and concentrated, while infection broadcast by droplet nuclei is more dispersed and dilute.” Crucially, the Wellses thought that the respiratory tract and other wet tissues could generate only large droplets. Their model thus held not only that airborne particles could be generated through the evaporation of larger droplets but that they could be generated only this way.
These kinds of firm boundaries later showed up in other aspects of the airborne–droplet distinction. For example, it is often held that droplets infect only the sinuses, mouth, and upper respiratory tract, while airborne particles infect the lungs. The reasoning here again owes largely to the simplistic Newtonian assumption that heavier droplets will fall out of the air too quickly to reach the lungs.
The neat airborne–droplet division became foundational to infectious disease control. But it was based on a set of assumptions that were poorly backed by limited measuring techniques. The Wellses, for example, drew many of their conclusions by indirect measurements, such as of the time it takes pure water droplets to evaporate. Other researchers in the 1920s through the 1940s attempted to measure droplet sizes using high-speed photography and glass slides held in front of the mouths of test subjects. These early studies did not find many small droplets — but this was because their instruments were largely incapable of detecting them.
Despite the pervasiveness of the airborne–droplet model, the science behind it was little updated until 1987, when researchers first measured exhaled droplets using an optical particle counter, capable of detecting particles of sub-micrometer size. They found that most of the particles were less than 0.3 micrometers across. These findings were confirmed in an even more systematic study in 1997, in which researchers used particle counters and electron microscopes to measure mouth breathing, nose breathing, coughing, and talking.
A 2009 article in the Journal of Aerosol Science reported the findings of a novel experiment in which test subjects’ heads were placed in a wind tunnel. The authors argued that this technique greatly increased accuracy, and allowed them to correct for the evaporation of droplets between inhalation and measurement. This article offered a yet more rigorous confirmation of the 1987 and 1997 findings: most particles produced in coughing, breathing, and speaking are less than 1 micrometer in diameter. They posited similar results for sneezing, which involves the same biological mechanisms of particle generation as coughing. And they provided this balletic image:
Droplet atomization from the respiratory tract arises from the passage of an air-stream at a sufficiently high speed over the surface of a liquid; tongues of liquid are drawn out from the surface, pulled thin and broken into columns of droplets.
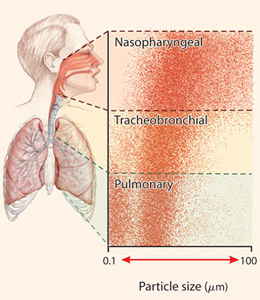
In sum, newer research shows that small particles are in fact produced directly from respiratory activity. It suggests that a few separate biological mechanisms are responsible, and that each mechanism has its own distinct size of particles that it mainly generates. But it also shows that a very wide range of sizes are ultimately generated, of which the large majority are small enough to float for some time and disperse over some distance.
Finally, the distinction between droplet and airborne transmission truly begins to crumble when confronted with the question of how different particles travel through the air. In April 2014, a team of researchers led by M.I.T.’s Lydia Bourouiba published the results of a study that used modeling and high-speed imaging to measure the dynamics of coughs and sneezes. Conventional approaches had modeled expelled droplets separately, as individual projectiles. But the researchers studied fluid-mechanical effects, and found strong interactions of the droplets with one another, as well as with the gas cloud expelled by a cough or sneeze.
Bourouiba’s team concluded that droplets, particularly small droplets, can travel much farther than was previously believed — some up to eight feet away horizontally and twenty feet vertically under the energy of their initial expulsion. This is more than far enough, the authors note, to reach most hospital ventilation systems. The paper, which argued that “the transmission mechanisms of even the most common respiratory diseases remain poorly understood,” prompted another researcher to comment that the study meant scientists “might have to rethink how we define the airborne respiratory aerosol size range.”
The picture of the dry explosion in the desert is a pleasing one. It’s useful for getting a quick grasp on how different mechanisms are at play in droplet transmission, how it’s likely that one mechanism (momentum) dominates the motion of heavy particles, while another (buoyancy) dominates light particles. But even this simple image does not support the sharp division of droplet versus airborne, for there is no bright line dividing particles that sink or float. And the physics of infectious transmission in the air turns out to be far more complex even than this, closer instead to what might be imagined by an educated guess: a great, big mess.
What has slowed recognition of the fact that the distinction between airborne and droplet transmission does not match reality? In part, the model has been kept alive through the addition in the literature of tweaks and adjustments, which today grow so thick as to resemble the “epicycles” that geocentrists used to bandage their model of the cosmos in its dying days. The medical community also understandably desires a transmission model that aligns with simple instructions on what kinds of precautions to take. These tendencies have delayed realization of how outdated the model is.
Some scientists have begun to recognize this state of affairs — particularly those whose focus is on protecting against disease transmission through the air. For example, environmental and occupational safety researchers Lisa Brosseau and Rachael Jones coauthored one of the September 2014 articles, mentioned above, warning of the possible transmission of Ebola through the air. Another of their articles, published in November 2014, argued that hospitals’ confusions in applying the conventional model arise from “artificial distinctions about particle size and transport distance.” This claim is echoed in many recent papers by other researchers. Brosseau and Jones argue that it is time for “a new paradigm,” and suggest replacing the notion of separate droplet and airborne modes of transmission with the broader concept of aerosol transmission.
This is a reasonable proposal, as the noun aerosol already encompasses droplet and airborne particles. An alternative, which would avoid the baggage associated with the term aerosol, would be aerial transmission, a relatively unused term that has the advantage of being well aligned with the everyday meanings of its words. (In this article, I instead use the phrase “transmission through the air,” which gains in accuracy what it sacrifices in concision.)
The emerging scientific picture of infectious transmission deals in graphs of particle sizes, generation rates, travel distances, and probabilities of penetration into various reaches of the respiratory tract, from which many meaningful trends can be discerned, but very few bright lines. So the new paradigm must be capable of dealing in shades of gray — and yet it must still allow for clear communication. It must permit more nimble changes in practices in light of evolving research — and yet must be capable of issuing in straightforward rules and recommendations that doctors and nurses can follow. As we will see, several researchers have proposals for just such a system.
So, then, to the looming question: Can Ebola be transmitted through the air? A full answer requires drawing on a number of sources: the physics and respiratory biology already discussed; limited animal modeling that may support the possibility; some research showing Ebola can persist in the environment for weeks under the right conditions; studies suggesting that the infectious dose of Ebola is very small, possibly on the order of tens of particles; tracing from outbreaks showing some cases with no known prior contact with other infected patients; and anecdotal data suggesting that health care workers who use airborne precautions are less likely to become infected. The details of this evidence, which is fragmentary and inconclusive at best, are presented in a supplement at the end of this article.
To say that the evidence on Ebola transmission through the air is inconclusive is to say only that: It cannot be definitively shown from current research either that Ebola is transmitted through the air or that it is not. The most reasonable interpretation of the evidence is that Ebola transmits through the air much less easily than tuberculosis and measles, but more easily than HIV, malaria, and plague. And this indeed may be a plausible explanation for some portion of documented Ebola cases: perhaps a fraction of a percent, perhaps a few percent, maybe several percent.
Epidemiology is the most reliable guide to true infection rates, but many researchers note that it may mask the true extent of a disease’s transmissibility through the air, since this mode of transmission is most likely to occur in the same close range where droplet and contact transmission occur. The decision to attribute any given infection to contact, droplet, or airborne transmission is often based just on the distance of contact — so in the epidemiological data, close-range infections are often assumed not to be airborne when in fact they might well be.
To put these findings in perspective, some researchers note that it is rather difficult to demonstrate transmission through the air of any disease, in part because of how minuscule the particles are — how difficult to capture, measure, culture, and detect. In a 2004 article in the New England Journal of Medicine, researchers Chad J. Roy and Donald K. Milton note:
The only clear proof that any communicable disease is naturally transmitted by aerosol came from the famous experiment by William Wells, Richard Riley, and Cretyl Mills in the 1950s, which required years of continual exposure of a large colony of guinea pigs to a clinical ward filled with patients who had active tuberculosis.
Is Ebola Like the Flu?
Roy and Milton argue that, since the few prominent diseases clearly known to transmit through the air — tuberculosis, measles, and smallpox — were beaten back decades ago by vaccines and antibiotics, “the impetus to understand the aerobiology of infectious diseases has faded.” They propose a new system, rather like the one biologists use to describe carnivores, in which a disease’s transmission through the air is classified as either obligate, preferential, opportunistic, or nonexistent. Tuberculosis is the only known obligate aerosol-transmissible disease — the only disease that must infect through the air. Measles and smallpox are preferential diseases. Presumably a great many diseases would be classified as opportunistic, though Roy and Milton name only SARS. Some researchers have hypothesized that Ebola is an opportunistic aerosol-transmissible disease.
During the outbreak, public health authorities repeatedly emphasized that Ebola is not a respiratory disease, which is true, but which falsely implies that transmission through the air is irrelevant to Ebola’s spread. Ebola patients still cough and sneeze and respire at normal rates. In fact, an October 2014 report from the WHO summarizing the first nine months of the outbreak in West Africa reported that coughing was a symptom among 30 percent of patients, and that coughing was a statistically significant predictor of which patients died.
Importantly, the risk of Ebola transmission through the air is more significant inside a health care setting than outside, particularly in developed countries. In part this is because the risk of becoming infected through direct or indirect contact is greatly reduced by the use of standard precautions, and so airborne risks become proportionally larger. But the risk is greater also in absolute terms: in health care settings, workers are in close range with patients for extended periods of time, and also sometimes perform medical procedures that generate infectious aerosols, a factor not relevant outside hospitals.
Perhaps the most compelling evidence about Ebola transmission through the air comes from health care settings. Although ethics prohibits testing the question in a controlled way, the fact that protective measures against Ebola vary so widely has created a kind of natural experiment, one that can demonstrate correlation if not causation. And the pattern is that Ebola workers who wear surgical masks often become infected, while those who wear respirators almost never do.
In the course of researching this article, I interviewed several occupational health and safety researchers and industrial hygienists, whose job it is to develop and promulgate standards for workplace settings that involve environmental hazards. Over and over again, in my interviews and in my review of their professional literature, I encountered the same question: As long as there’s uncertainty, why not use respirators? Why not err on the side of safety?

 From top to bottom: a surgical mask; an unpowered N95 respirator; and a powered air-purifying respirator (PAPR). Images from Kimberly-Clark, Moldex, and 3M, respectively. Fair use.
From top to bottom: a surgical mask; an unpowered N95 respirator; and a powered air-purifying respirator (PAPR). Images from Kimberly-Clark, Moldex, and 3M, respectively. Fair use.
There are, it turns out, downsides to respirators. Surgical masks are very simple to take on and off. Many respirators, though not all, require fit testing, and the ones that include hoods can take a few minutes to get into and out of. Ones that require a seal around the face are not effective for workers with facial hair. Also, ones that cover the face must be properly doffed if workers need to, say, scratch an itch — a deliberate restriction, as touching the eyes or face during treatment is a prime way to get infected. Industrial hygienists I spoke with argued that tolerating these minor irritations is simply part of the job description, just as it is for divers and astronauts. “You get used to it,” one told me.
The other common consideration is cost. The strongest powered respirators needed to treat an Ebola patient are available for a few hundred to a thousand dollars. There are also recurring costs for batteries and replacement filter cartridges, on the order of a few hundred dollars per year. Powered respirators also require staffing to clean and maintain them, though these costs are mostly fixed rather than marginal.
The most common type of respirators, known as N95 respirators, look at first glance somewhat like surgical masks, but they still offer more respiratory protection than surgical masks — which provide virtually none. N95-rated respirators can be purchased in hardware stores for a few dollars. These aren’t the ones typically used in health care, but a box of N95s approved by the CDC’s National Institute for Occupational Safety and Health can be bought on Amazon for a dollar or less per mask — though they are meant to be disposed of after use. Even though N95s do not require the same kind of staffing costs for maintenance, they still incur administrative and training costs for the annual fit testing; one California hospital administrator, in a National Academy of Sciences publication, pegged the time as 30 minutes per employee annually.
But powered respirators — those with a powered filter or an isolated air tank — are considered the gold standard of effectiveness. And the majority view among the experts I spoke with and in the literature is that they are also more comfortable than unpowered respirators, and even than surgical masks: powered respirators don’t stick to the face, they don’t obstruct breathing, they generally don’t require a fit test, and the built-in ventilation keeps face shields from fogging and the enclosed air from becoming uncomfortably humid with breath. But hospital administrators are reluctant to sink the equipment costs. Meanwhile, health care workers and administrators alike often prefer not to bother with the equipment unless a compelling need has been demonstrated.
Of course, the expenses associated with respirators must be pitted against the costs — monetary, institutional, and psychological — of doctors and nurses contracting diseases from their patients. But a direct comparison is actually difficult: the upfront costs and hassles of using the equipment are immediately seen and felt, while tracing a worker’s infection back to some particular laxity of protection will always be obscure. Given the difficulty of pulling apart the tangled, invisible web of infection factors in a hospital, it is easy to say that infection risk is just part of the job, or to assume reflexively, as CDC Director Frieden did in 2014 and as the agency still maintains today, that a particular infection must have occurred because of a “breach in protocol,” rather than a laxity in the protocol itself.
But the widespread reluctance by the medical leadership to adopt respirators as the standard of care for Ebola ultimately had less to do with concrete costs than with certain intangible factors. It is to these that we turn next.
Ebola has no greater friend than fear,” said Samantha Power, U.S. ambassador to the United Nations, in late October 2014. Michael Kinzer, a CDC epidemiologist, took the idea a step further in remarks to news media in Guinea: “Ebola’s not transmitted by the air. Fear and ignorance are transmitted by the air.” WHO Director General Margaret Chan agreed, saying, “rumors and panic are spreading faster than the virus, and this costs money.”
This line — that fear of the virus was a contagion, perhaps even the real contagion — too cute by half, was picked up and run with by journalists. With numbing inevitability, the ghost of FDR was summoned in concert from a thousand news desks around the world, reanimated as a public health scold in op-ed headlines about fear itself.
With something like Thomas Frieden’s mixture of reassurance and condescension but a stronger dose of the latter, the news media rushed to douse the flames of panic. Explainer-journalism site Vox released an infographic with a single question: “Have you touched the vomit, blood, sweat, saliva, urine, or feces of someone who might have Ebola?” The only option was no, and the only conclusion, “You do not have Ebola.”
The wave of anti-panic journalism crested on October 23, when a New York City physician who had recently returned from treating Ebola patients in West Africa, and who had been traveling around the city for two days when he may have had early symptoms, tested positive for the virus. “You are not going to get Ebola, New Yorkers. No one you know is going to get Ebola. Have a good night,” tweeted Ben White, a Politico economics correspondent. Vox’s journalists doubled down on their infographic, even though in the time since it was published three American health care workers had tested positive for Ebola.
Following like clockwork were the psychological diagnoses. An NPR science correspondent aired a segment seeking to understand why public fears over airborne transmission persisted despite sustained official pronouncements to the contrary; the segment did not waver from this premise even after noting researchers who claimed that those official pronouncements were not well founded in fact. Similar pieces searching for the psychological sources of an irrational fear were published by Time, the New York Times, the Atlantic, the Washington Post, the New Republic, Psychology Today, the Bulletin of the Atomic Scientists, and other outlets. In a widely circulated Wired article, writer Maryn McKenna attempted to coin the term of the moment: “Ebolanoia.”
Few of these articles made note of the scientists pointing to evidence for more serious concern, except in a perfunctory way. And all of these articles were published after the infection of the first Dallas nurse.
A compendium of damages inflicted during the Ebolanoia outbreak was compiled by McKenna. The most notable cases:
● An assistant principal at a North Carolina middle school was required to stay home for 21 days after returning from a trip to South Africa, a country far from the outbreak.
● Some parents in a Mississippi town pulled their children out of a middle school after its principal attended his brother’s funeral in Zambia, which is far from the main outbreak (although there was a simultaneous, independent Ebola outbreak underway in neighboring DR Congo).
● A congressman best known for shouting at President Obama during a State of the Union address warned that Ebola could be used as a bioterrorism weapon and proposed travel bans and border closings.
● A woman showed up for a flight wearing a hazmat suit.
● A suggestion that Dallas adopt a public no-touching policy was floated by a third-tier candidate for the U.S. House of Representatives who a few weeks later would land five percent at the ballot box.
These were excessive reactions, to be sure, though some were not even clear instances of paranoia. For example, the National Institutes of Health in fact lists Ebola as a Category A biodefense priority pathogen — a rating so exclusive as to include only the hemorrhagic fevers, anthrax, smallpox, plague, botulism, and tularemia. The defense community’s concern is not simply theoretical: The Japanese doomsday cult Aum Shinrikyo, which killed a dozen people and injured over a thousand in a deadly sarin-gas attack on the Tokyo subway in 1995, also attempted in a very rudimentary way to obtain Ebola cultures for weaponization.
As for the rest, these cases were at worst instances of modest overreaction by people in positions of minor authority. This, along with cries of falling skies from those who offer this same reliable forecast no matter what the weather, constitutes the documented extent of what Time dubbed “Our Collective Ebola Freak-Out.” This was the high-water mark of panic. This, out of a nation of 320 million people.
Keep tabs on the average incidence of foolishness in hamlets across this country prompted by any given subject of national attention over an eight-week span, and marvel that Ebola spurred as little as it did. As the Atlantic’s Derek Thompson noted, polling showed that the number of Americans who thought they or a family member were likely to get Ebola was half the number who believe in witches. And yet this supposed hysteria was considered so dangerous that some public figures deemed stamping it out a greater imperative than frankly discussing the risks.
The notion that fear posed a risk comparable to the virus itself held sway even in the medical and scientific communities. For example, when four environmental engineers, led by the University of Pittsburgh’s Kyle Bibby, offered a review of the scientific literature on Ebola in December 2014, other scientists criticized them for irresponsibly stoking the public’s fears. Bibby and his coauthors highlighted some gaps in the knowledge of Ebola’s transmission via surfaces, sewage, and water supplies. “While environmental exposure is not the dominant exposure route,” they wrote, “available data suggest that it is imprudent to dismiss the potential of environmental transmission without further evidence.”
But in a response that appeared in the same publication, Environmental Science and Technology Letters, environmental engineer Daniele Lantagne and epidemiologist Paul Hunter scolded, “As scientific professionals, it is our responsibility [to] give a balanced presentation of scientific knowledge to the public.” Lantagne and Hunter asserted that Bibby and his coauthors presented a one-sided picture intended to give the impression of a definite risk — a mischaracterization of the Bibby paper, which repeatedly described how poorly the risk of waterborne Ebola transmission is understood and suggested that the risk be treated with some precaution. Lantagne and Hunter wrote that Bibby and his coauthors, by suggesting that CDC and WHO guidelines might be inadequate, “could contribute to the culture of fear around Ebola.”
A similar argument arose around the article, published online in September 2014 in the International Journal of Nursing Studies, by Australian researcher Raina MacIntyre and four coauthors urging that Ebola workers wear respirators. In a response, WHO-affiliated public-health expert José Martín-Moreno and two coauthors argued that respirator usage is not supported by evidence. They concluded that workers who wear biohazard suits
undermine risk communication messaging — so crucial to control transmission outside of hospitals — by suggesting that the precautions recommended to local populations are inefficacious.
Just a few weeks earlier, Martín-Moreno and his coauthors had written a commentary for The Lancet, a leading journal, warning against “excessive precautions” by Ebola workers. Respiratory protection, they argued, is “unaffordable for countries that are the most affected.” Moreover, using it “suggests that the only defense is individual protective equipment, which is inaccessible to the general population,” and this “might contribute to the panic in some communities.” Using respirators also “reinforces the view that some lives are more valuable than others.” Therefore, it is crucial to sustain “a consistent message that the disease is essentially transmitted through direct contact.”
This attitude was not isolated. Thomas Fuller, a founding member of the pandemic planning team at the American Industrial Hygiene Association, told me about conversations he had with a hospital physician who had treated patients during the 2002 outbreak of SARS. When infection-control authorities assured nurses that they did not need any respiratory protection, some took it upon themselves to wear N95 respirators anyway, and were chided by doctors that this would scare patients.
In an article called “Ebola Fever,” published in August 2014 in the Annals of Internal Medicine, Harvard population-medicine professor Michael Klompas and three coauthors warned hospitals against “the temptation to maximize precautions that exceed CDC recommendations.” Using respirators and other such “extra gear,” they wrote,
inflates patients’ and caregivers’ anxiety levels, increases costs, and wastes valuable resources. More insidiously, requiring precautions that exceed the CDC’s recommendations fans a culture of mistrust and cynicism about our nation’s public health agency.
As health care professionals, we strive to provide evidence-based care driven by science rather than by the media or mass hysteria.
The implication of these arguments was clear: for scary diseases on which scientific evidence is inconclusive, the medical community should err not toward protecting health, nor toward honest communication, but toward assuaging the public’s psychic unease.
It is difficult to know the extent to which fear of fear itself may have influenced the recommendations of the CDC and the WHO regarding the necessary precautions against the spread of Ebola. For that matter, it is difficult to tell what their decision-making processes were at all — what was the reasoning for disregarding the tentative evidence for transmission through the air; why the lack of clear evidence one way or the other did not lead to a precautionary stance; what systematic risk analysis, if any, was used; and what was the reasoning for the CDC altering its guidelines on October 20, 2014.
We do know, however, from descriptions in publicly available documents, that officials at both the CDC and the WHO were aware of the very inconclusive nature of many key aspects of the research related to protection against the transmission of Ebola through the air. In reading through many hundreds of such documents, the limited nature of the evidence is often acknowledged — usually with a formulation like “there is no evidence that….” And yet almost never did I encounter any accompanying suggestion that the inconclusiveness itself might warrant a precautionary approach, or that resolving these many questions ought to be a research priority. Those suggestions came only from researchers outside the two organizations. And there are indications that the CDC and WHO sought to discourage discussion of research that conflicted with approved views.
One such case involves Raina MacIntyre, a researcher mentioned above. An epidemiologist by training, MacIntyre is head of the School of Public Health and Community Medicine at the University of New South Wales in Sydney, Australia. On August 11, 2014 — when the epidemic in West Africa was already the largest Ebola outbreak in history — MacIntyre e-mailed three members of the steering committee that crafted the WHO’s April 2014 guidelines for prevention of respiratory infections. Those guidelines had recommended that workers treating patients with acute respiratory infections use surgical masks instead of even unpowered N95 respirators, on the basis that studies had found no difference in effectiveness between surgical masks and unpowered respirators, and that surgical masks are cheaper and more comfortable.
In her original e-mail — MacIntyre has shared the entire exchange with The New Atlantis — MacIntyre noted that the guidelines cited only a single randomized controlled trial comparing the effectiveness of masks with unpowered N95 respirators in a health care setting. But it ignored the three other known trials, which she had conducted. MacIntyre’s trials, involving a total of 3,110 health care workers — compared to 446 in the single previous trial — had shown a strong advantage for respirators over surgical masks in preventing infections from several respiratory illnesses.
Seven days later, Sergey Eremin, a medical officer with the WHO’s Department of Pandemic and Epidemic Diseases, replied to MacIntyre that he and his colleagues were in “emergency mode,” but promised a quick and substantive response. MacIntyre replied the next day, noting that her trials showed respirators to be more protective than surgical masks even against viruses that were not considered conventionally airborne. The WHO’s guidelines specifically for Ebola, newly updated that month, recommended only surgical masks. MacIntyre concluded, “I am deeply concerned about the risk to [health care workers] and feel this is urgent.” Dr. Eremin did not reply.
On September 4, MacIntyre delivered a public lecture outlining the flaws in mask recommendations and warning of an “urgent need” for the CDC and WHO to strengthen their guidelines. On September 9, her article in the International Journal of Nursing Studies was published online; as noted above, it made the case for the use of respirators by health care workers dealing with Ebola.
More than a month later, when the CDC updated its own guidelines to include respirators, MacIntyre e-mailed Eremin; she pointed out the change in CDC policy and again asked for a response from the WHO. Eremin finally replied the following week with a lengthy e-mail mainly restating the substance of the WHO report. He also noted that the WHO guidelines drew largely from a 2011 literature review, and that MacIntyre’s three studies were published later. However, the WHO guidelines themselves were released in April 2014, three months after the latest of MacIntyre’s studies. Eremin stated that her studies “were included in our preliminary analysis of additional studies, but we have not conducted a new systematic review of the literature on this topic.”
Eremin then argued that MacIntyre’s trials suffered from methodological shortcomings, and concluded with suggestions for improvements to future studies. His e-mail did not remark on the CDC’s upgraded guidelines. It should be noted that Eremin was an author of both 2014 versions of the WHO Ebola protection guidelines, from August and December, the latter of which remains in effect as of this writing. Both recommended only surgical masks. Eremin did not respond to requests for comment from The New Atlantis.
In another disconcerting case, in the fall of 2014, the WHO rejected an offer of assistance from Linda Forst, a University of Illinois at Chicago (UIC) professor who regularly consults with American and international health agencies, and who runs a WHO collaborating center. Simply declining the offer of assistance would have been unremarkable, but the WHO declined on the basis that one of Forst’s colleagues, Lisa Brosseau, has received funding from 3M, which manufactures medical equipment. This account was confirmed by both Brosseau and Forst.
While it is true that WHO policies require collaborating centers to have no real or perceived conflicts of interest, Brosseau told The New Atlantis that she has not received support from 3M for several years and that when she did she was at a different university and it was a small fraction of her funding. (3M declined to confirm the funding amount.) Moreover, publications indicate that the center has remained active in new collaborations with Forst since Brosseau joined UIC, indicating that the WHO’s concern over this ostensible conflict has been selective. The WHO has declined to comment on these details.
It may be that the conflict of interest in this case lies in the other direction. As noted earlier, Brosseau has been outspoken in warning that Ebola may be transmitted through the air and that the WHO and CDC should recommend powered respirators. She is a known quantity among the WHO top leadership. The flimsiness of the justification the WHO used to decline the assistance of Brosseau’s colleague Forst invites speculation: perhaps the rejection was the result of Brosseau’s substantive views. The WHO has again declined to comment on this question.
Another troubling episode involves the literature review published in the journal mBio in February 2015. As noted above, this paper concluded that “It is very likely that at least some degree of Ebola virus transmission currently occurs via infectious aerosols.” The authorship on the paper is a who’s-who of the most recognizable and respected names in the study of hemorrhagic fevers and infectious disease, including several individuals who were deployed to West Africa, and some who directly treated patients during the outbreak. (Brosseau, it should be noted, is among the authors.) Devoid of any policy suggestion, the mBio article is purely an analysis of the scientific literature, written in careful, tentative language.
And yet, one of the authors has taken the unusual step of asking to have his name removed from the paper: Pierre Formenty, a respected field epidemiologist who works at the Department of Epidemic and Pandemic Alert and Response at WHO headquarters in Geneva. An mBio spokeswoman stated that this is the first authorship-removal request in the journal’s five-year history. Formenty did not respond to our repeated inquiries asking for comment, but a WHO spokesman says that Formenty asked to have his name removed from the paper because “he had not [had] time to read, review and edit the text” before it was published. The lead author on the paper, Michael Osterholm of the University of Minnesota, confirmed that Formenty had asked to have his name removed, and that, because of a miscommunication, Formenty had not approved his authorship prior to publication, though he was involved in drafting the paper.
But multiple sources with direct knowledge of the situation, who have asked to remain anonymous, tell The New Atlantis that Formenty’s request to have his name removed from the paper was the result of pressure from the highest levels of WHO leadership disapproving of the paper’s conclusions, which were not fully in concert with official WHO positions on Ebola. When asked, the WHO spokesman did not address the question of whether Formenty was pressured by WHO leadership.
Several researchers I spoke with — although by no means all — described patterns of discouragement from the CDC and the WHO against open discussion of airborne disease risks. Employees showed reluctance in professional settings to speak openly of research that contradicted official statements, and pressure was exerted behind the scenes to downplay the possibility of disease transmission through the air. And there was a general sense among many researchers that the organizations’ statements and stances were sometimes determined more by political concerns than by frank readings of research. These patterns were said to have existed for years, and to have pertained to discussions not just of Ebola but of several other diseases.
The New Atlantis has filed two Freedom of Information Act (FOIA) requests with the CDC in conjunction with this article. One request is for copies of e-mails, sent to or from certain CDC employees during the outbreak, discussing airborne transmission and respiratory protection. The other is for internal documents, other than e-mail, discussing the same. These FOIA requests remain outstanding as we go to press.
Whether the CDC’s and the WHO’s failures in protecting health care workers were knowing, or even deliberate, matters a great deal. But whatever the case, it is clear that the organizations failed to solicit enough challenging opinions — that they were content to defend encrusted ideas on the grounds of limited evidence rather than to vet doubts aggressively. The contrary evidence was not difficult to find, at least for those who wanted to see it — and many respected researchers were working to get it seen.
The CDC’s rationale for finally upgrading its respiratory protection guidelines on October 20, 2014 remains obscure. Their few public statements on this matter make little coherent sense, and give the impression of invoking strictly scientific rationales to justify decisions made for many other reasons. The CDC media office, in response to a request for comment on why the agency changed its guidelines, said, “The recommendations that CDC released in late October provided more detailed guidance for healthcare workers who might be exposed to Ebola.” Strangely, this characterization and the rest of the response did not acknowledge that the guidelines had been substantively changed at all, only clarified.
Aside from the claim that respirators were not necessary, the other common argument against them was that they may actually be more hazardous than surgical masks. The CDC repeatedly invoked this idea in the ten days between the infection of the Dallas nurses and when the agency finally upgraded its recommendations. The WHO, which has not yet changed its guidelines, continues to make this specious claim. A WHO spokesman, reached by e-mail, claimed that, because Ebola is a droplet disease, respirators do not offer stronger protection than surgical masks, while “Using a poorly fit respirator in a hot and humid environment may be even more dangerous than using properly a structured non-collapsible surgical mask.”
It is of course well known among the medical community that the act of removing hoods, masks, double gloves, and suits carries its own risks when performed improperly: It presents a number of opportunities for infectious fluids successfully trapped on the exterior of the equipment to now make contact with the body. But it’s the task of manufacturers to innovate more foolproof equipment, of safety researchers to innovate procedures to reduce these errors, and of public health organizations to disseminate these new standards. The CDC itself stopped invoking the idea that respirators might be more hazardous than surgical masks after it upgraded to recommending respirators. And in a video released for health care workers a few weeks later, the CDC reassured that respirators “are used every day in U.S. hospitals to safely care for patients.”
Two final points are worth noting. First, the CDC and the WHO both formerly recommended respirator usage for workers providing routine care to Ebola patients. A set of 1998 guidelines, prepared and issued jointly by the two organizations, recommended the use of respirators for all workers near patients even suspected to have hemorrhagic fevers, including Ebola. Surgical masks were recommended only when respirators were not available. These and other measures were justified on the grounds of preventing droplet and airborne transmission.
It is unclear why these guidelines were changed. A WHO spokesman said that “the old 1998 guidelines [were] issued when [less] was known about Ebola than we know now. Probably, the authors extrapolated some inconclusive data coming from lab animals. The respirators [were] clearly an attempt ‘to err on the safe side.’” The respirator recommendation was dropped with a 2008 update to the guidelines.
Then there is the fact that the CDC’s guidelines for laboratory rather than health care settings put Ebola on the list of pathogens requiring the most stringent possible level of protection. This designation, Biosafety Level 4, is very exclusive — only a few diseases merit this protection level. It requires the use of powered respirators, among a number of other strict measures. A current CDC training guide on the Biosafety Levels explains:
The microbes in a BSL-4 lab are dangerous and exotic, posing a high risk of aerosol-transmitted infections. Infections caused by these microbes are frequently fatal and without treatment or vaccines. Two examples of microbes worked with in a BSL-4 laboratory include Ebola and Marburg viruses.
As Raina MacIntyre has documented, nearly all public health agencies in the world have much more stringent safety standards for dealing with Ebola in laboratories than in treatment settings. On the one hand, this state of affairs, which MacIntyre describes as “a double standard,” may owe simply to practicalities: it is much easier to adhere to rigorous safety standards when working with inert samples than with live patients. On the other hand, as MacIntyre points out, this difference actually means that health care workers are at much greater danger in the first place — working with live patients means they are far more likely to be exposed to infectious fluids and aerosol-generating events, and the environmental controls against these modes of transmission are typically much weaker in hospitals than in research labs.
It is unclear, then, why the CDC insisted during the Ebola outbreak that ordinary hospitals were capable of safely treating Ebola patients — why it did not recommend that confirmed cases be transferred as quickly as feasible to high-containment units like the one at Emory University, which successfully treated four cases with zero patient mortality and no infection of hospital staff. And it is unclear why the CDC did not recommend that, barring such transfers, health care workers, even if they could not meet Biosafety Level 4 standards, at least use the stronger protective measures already available to them.
Journalists and government investigators have uncovered a range of institutional problems at the CDC and the WHO since the Ebola outbreak began, and while an in-depth analysis is beyond the scope of this article, it is worth highlighting a few items.
Sheri Fink, in a report in the New York Times, described bureaucratic dysfunction that hampered the WHO’s early response to the Ebola crisis, including a “balkanized hierarchy” in which various offices “jockeyed for position.” Fink also remarked that “the whims of donor countries, foundations and individuals also greatly influenced the WHO’s agenda”; for example, as she noted in an NPR interview, the Bill and Melinda Gates Foundation now gives more money than any single country, “and they get to choose the priorities … as long as it fits within the WHO’s mandates.”
A similar, if much less pronounced, state of affairs may hold at the CDC. In a recent report in the British Medical Journal, Jeanne Lenzer revealed that the agency receives millions of dollars in funding from private individuals, philanthropic foundations, and corporations — including medical manufacturers. Although that money makes up a tiny portion of the overall CDC budget, Lenzer cites numerous instances in which the agency issued recommendations that seem to have directly benefited its corporate donors.
Meanwhile, investigations by the Washington Post and the New York Times have described bureaucratic friction between the CDC and the WHO. And a special panel convened to assess the WHO’s response to the outbreak has reported on several “organizational failings.” It found that the WHO “does not have an organizational culture that supports open and critical dialogue between senior leaders and staff or that permits risk-taking or critical approaches to decision-making,” and “does not currently possess the capacity or organizational culture to deliver a full emergency public health response.”
For present purposes, however, what is most interesting is the tendency of both organizations to put optics — public perceptions and political correctness — ahead of wise policymaking. In the case of the WHO, this is not a novel critique; Steven Menashi argued in these pages more than a decade ago that “the WHO attempts to pass off its political preferences as scientific expertise” (see “The Politics of the WHO,” Fall 2003). In March 2015, the Associated Press uncovered e-mails showing that WHO officials delayed declaring a global emergency over the Ebola epidemic because of “worries that declaring such an emergency — akin to an international SOS — could anger the African countries involved, hurt their economies, or interfere with the Muslim pilgrimage to Mecca.”
This is not the only instance in which the WHO has expended some of its apparently critically limited attentional resources on avoiding hurt feelings. In May 2015, as the outbreak was still gripping West Africa, the WHO called for changing practices in naming future diseases, so as not to include names of places, people, or industries, a practice that has “had unintended negative impacts by stigmatizing certain communities or economic sectors.” Among the diseases mentioned were Creutzfeldt–Jakob and Chagas — both named after their long-dead discoverers — as well as monkey pox and MERS (Middle East Respiratory Syndrome). Not mentioned in this statement was Ebola, which is also named for a place: the Ebola River in the DR Congo, where the disease was first identified. The WHO recommendations then become even stranger: the organization urges avoiding “terms that incite undue fear” when describing diseases, specifically calling for a prohibition of the words “fatal,” “epidemic,” and “unknown.” This coming at a time when the death toll from Ebola stood at 11,000 and counting, from an outbreak whose seriousness the WHO had been almost pathologically slow in acknowledging.
For the CDC and the broader U.S. government, no part of the Ebola response was more politically fraught than the debate over whether to require quarantines for health care workers returning to the United States after treating Ebola patients in West Africa. To be sure, quarantines are a complicated policy measure, with considerations for and against that are difficult to quantify, much less to reconcile. There are good reasons to use them sparingly. But Ebola workers were a high-risk group by any reasonable estimation — particularly in West Africa, where infection controls were generally poor — and the duties of a medical professional are rather different from those of an ordinary citizen. From many quarters, including President Obama, the argument was made that quarantines — even just for workers with low-level symptoms, as in the New York and New Jersey policies that the Obama administration aggressively sought to quash — would scare workers away. No evidence was offered for this claim.
Much more peculiar than the claim of a material disincentive — the hassle of three restricted weeks — was the claim of a moral disincentive. White House press secretary Josh Earnest referred to quarantines as “outright disrespecting health care workers.” Thomas Frieden warned of turning workers into “pariahs.” Samantha Power urged that returning workers be treated “like conquering heroes and not stigmatized for the tremendous work that they have done.”
Perhaps the most surprising person to voice this idea was Craig Spencer, the doctor who himself became ill with Ebola after returning from West Africa and traveled freely around New York City for two days with possible early symptoms before he was isolated. Despite being the walking demonstration of the case for quarantines, Spencer repeated the “pariah” argument against quarantines in an editorial after his recovery.
It is no exaggeration to call the work of Dr. Spencer, and others who risked their lives to help Ebola-ravaged nations, heroic, especially because they volunteered for the job. But it is passing strange to suggest that it disrespects medical workers to ask them to follow the primary mandate of their profession — first, do no harm. Nobody regarded as stigmatizing the Pentagon policy of quarantining for 21 days all personnel returning from Guinea, Liberia, and Sierra Leone, regardless of their symptoms or proximity to infected patients. Nor, for that matter, was it considered an insult to the heroism of the Apollo astronauts when, to guard against the remote possibility that they had encountered unknown pathogens on the Moon, they were upon returning to Earth placed in quarantine for 21 days.
The leadership of the Obama administration and the CDC could have directed their considerable public influence toward making this point. Little political energy would likely have been needed to ensure that quarantines functioned as a free vacation — a home stay with overtime pay, no expenditure of work leave, no other professional penalty, perhaps even a civilian medal awarded and a nice gala held for them all in recognition of their uncommon civil service. Even had a carefully risk-oriented evaluation judged quarantines unnecessary in this particular case — and no such evaluations were in evidence — it was peculiar to see political leadership claim helplessness before social forces that it suggested were unalterable even as it sought to reinforce them through its very words.
A similar attitude was at play when it came to the question of whether Ebola could be transmitted through the air. It was the attitude that led public health officials to argue, perversely, that better protection was somehow hazardous — that workers should avoid it rather than get training to ensure they used it properly. It was the attitude that led some to argue, in leading medical journals, that protection more robust than recommended by the CDC and the WHO should not be used, indeed that the possibility should not even be discussed, for this would scare people and undermine confidence in the effort.
The leaders who followed this approach believed they had the best interests of the public in mind. They were dealing with the perennial, unenviable task of protecting health and safety without sacrificing other goods — the dignity of patients and workers, the maintenance of political and economic stability, trust in public health officials. But we should be clear about the particular balance that they chose to strike between these goods.
It might seem that the belief that health care workers and the public are too psychologically fragile to handle open discussion of the evidence of infectious diseases is a kind of paternalism. But that isn’t quite right. For paternalism — laws compelling seatbelt use, taxing tobacco, restricting the size of soda cups — is an attitude willing to sacrifice liberty for the sake of health and safety. No, in the attitude at play here, safety is just the thing being sacrificed. And not some lofty perfection of safety, but specific protective measures, minimally burdensome and already widely employed, against a highly lethal and poorly studied disease.
This is a paternalism concerned with hygiene not of body but of speech and thought. It is not in fact paternalism but authoritarianism, whose interest is in calmness and order first, health and safety second, rational dissent last. It is the reverse ordering of an open, scientific society.
Here, then, we see the stakes of the Ebola gamble. The question at issue, the uncertain outcome, was whether Ebola transmission can occur through the air. Prevailing members of the public health leadership bet that the answer was no. The perceived gains were the preservation of order and calm; the savings of equipment, training, and bureaucratic expenses by sticking to the current practices; and, for some, the gratification of posturing as siding with science over hysteria. The potential losses were people’s lives.
The sad irony of this gamble is that it established a false choice and then lost both sides, putting workers at needless harm without even achieving the desired aims. By downplaying concern and silencing criticisms, public health officials weakened their response and lost some of the public’s trust. Their belief that honesty, credibility, and vigilance could not be had together was a self-fulfilling prophecy.
Many of the problems described in this article arise from complicated institutional dysfunctions; their ultimate resolutions will not be scientific but cultural and political. We might expect the CDC and the WHO to be the most credible authorities on medical science; the best sources of systematic risk analyses of protective guidelines; and leaders, to some extent, in directing research needs. As Steven Menashi wrote in these pages of the WHO, its “usefulness lies precisely in its ability to bring scientific evidence to bear in political disputes that often lose sight of facts on the ground.” Instead, the impression of the CDC and the WHO — conveyed by many researchers I spoke with, by the scientific literature, and by the strange path of the agencies’ actions — is of a hidebound medical culture, erring toward protecting reputations, not in front of events but fighting a rearguard action, invoking scientific doubt to cover for weakness of leadership.
These problems are exacerbated by the natural tension between the different evidentiary standards that apply to science and medicine. Science is interested primarily in truth, and refuses to offer firm conclusions until evidence is overwhelming; but medicine is interested primarily in action, and doctors must decide based on whatever evidence is available. Medical authorities not only demonstrated undue reluctance to change practices in the light of partial evidence, but often tried to pass their reluctance off as purely scientific. This confusion resulted in not only a weakened medical response but a less than forthright reading of the science. Frankly confronting the difference between the evidence necessary for scientific conclusions versus that necessary for medical actions will be central to avoiding these kinds of mistakes in the future.
In light of these problems, then, a few themes for reform suggest themselves:
State the confidence levels. Public health literature will occasionally state that particular protection recommendations are based on “high quality evidence,” “very low quality evidence,” and so on. In recent years, basic qualitative descriptions of confidence have been a welcome addition to many parts of everyday life — including weather forecasts and various aspects of personal health care — and they should become standard in public health contexts as well.
Encourage diversity in protective practices. The arguments employed to discourage health workers from adopting protections beyond CDC recommendations are spurious and counterproductive. They result from the mistaken idea that the organization’s authority cannot tolerate deviation.
It may be wise, then, for guidelines not only to permit but to formalize dual standards: recommended and discretionary protections. This bifurcated approach would be particularly valuable for contexts in which risk certainty is low but severity is high. It would legitimize the present reality that many individual hospitals and health care workers prefer stronger safety than the guidelines. It could also provide useful natural experiments, if studies are enacted to track the relative outcomes of the varying practices.
This approach would also provide useful political benefits to the CDC — reducing pushback about still-disputed recommendations, averting the inevitable criticisms that arise from shifting between one side and the other of an all-or-nothing stance.
Improve risk-analysis techniques. Risk analysis is designed precisely to deal with resolving uncertain information into definite action. Especially promising is an approach called control banding, already used in some industrial and medical contexts. Control banding breaks aerosol hazards down into a few basic “control” factors — the level of ventilation in the treatment room, the amount of time the worker expects to be treating the patient, the rate of aerosol generation from the patient, and the severity of the disease itself — and issues a decision about the necessary level of respiratory protection. It forces guesses to be made even where data is limited, rather than permitting deferral to some indefinite future when data will be available, and it simplifies and clarifies complex judgments about risk. Canada’s Robert-Sauvé Occupational Health and Safety Research Institute has created a shiny online tool that can be used to apply the control banding process to any infectious disease. This simple, elegant concept could have worked wonders if it had been employed by the CDC in crafting its guidelines regarding the use of protective equipment.
Industrial hygienists also have a broader array of techniques that could improve the prevailing medical approaches. Quite unlike the default stance in many health care contexts, hygienists assume situations hazardous until proven safe. This approach is not one of boundless precaution, but involves a series of analytic techniques for quantifying risks and establishing appropriate controls.
Although personal protective equipment is the focus of much of this article, it is only one aspect of a broader set of safety standards. Indeed, industrial hygienists place it last in their hierarchy of control measures. Lisa Brosseau explains that it is “the last and least desired form of protection — because it places the burden of control on the employee and has the greatest chance of failure.” So while control banding and similar techniques are useful for recommending personal protection levels, they are just as useful for giving a sense of the relative safety gains to be won by hospitals adding stronger ventilation, better isolation, faster rotation of health care workers to decrease their exposure time, and so on.
Central to these kinds of analyses is a basic distinction that was often forgotten during the outbreak. The claim was frequently made that Ebola is less dangerous than diseases like measles because it is harder to get. While this is true — by one standard metric, measles is nine times more contagious — it ignores the fact that Ebola is far more lethal: you are hundreds of times more likely to die from Ebola infection than from measles. This difference — between the likelihood and severity of a risk — must be acknowledged.
Greater candor in risk communication. Researchers Peter Sandman and Jody Lanard have studied decades of government responses to public crises, and identified common patterns of leadership failure. In case after case — Hurricane Katrina, terrorist attacks, numerous disease outbreaks — agencies withheld and downplayed risks out of the misplaced belief that doing so would avert public panic. But in fact this only fuels public distrust, resulting in further condescension by authorities — a spiral that deepens until the crisis has passed. Sandman and Lanard argue that fear can be a rational response to novel, poorly understood threats, and that what seems like overreaction is really part of a natural “adjustment” that can induce vigilance. This fear must be met, they argue, with information, not evasion. The Ebola response plays out as a textbook example of the patterns they identify, and their recommendations — be as honest and forthcoming about uncertainty as possible, and “treat the public like grown-ups” — should be required reading for public health leaders.
Prioritize the development of protective equipment, as well as studies of its effectiveness. Much of the confusion and contention in the debate over respirators owed to the fact that relatively little systematic research has been done to establish the effectiveness of different kinds of protective equipment in guarding against actual contagions. What research there has been has largely been conducted by occupational-safety researchers outside the public health agencies, which have apparently resisted the researchers’ conclusions.
There is much innovation left to be done. For example, one of the common sources of resistance to powered respirators is the hassle of sterilizing them after use, which normally means thoroughly rinsing them with disinfecting fluids. There may be more effective methods. Ultraviolet light is already commonly used for disinfection in a wide range of industrial contexts, as well as in some hospital ventilation systems. There has been some research in using UV light to disinfect respirators, including one contract awarded by the FDA. This technology could make respirators easier and cheaper to deploy.
Strengthen our understanding of the mechanics of infection through the air. The basic biomechanics of disease transmission through the air has been relatively neglected for much too long. The urgency of changing this state of affairs is clear. Perhaps the most frustrating part of wading through the literature in this area is to encounter over and over public health authorities squabbling with outside researchers — all under the thinly polite guise of scientific language — over how to interpret a woefully impoverished set of evidence. The researchers point to the gaps as presenting a problem, the authorities point to the gaps as presenting no answer, and little happens.
Here the Department of Defense has again demonstrated more systematic leadership than many public health agencies: the Defense Threat Reduction Agency posted a request for funding proposals in 2014 on research to counter Ebola. It specifically notes that “There is minimal information on how well filoviruses” — the family to which Ebola belongs — “survive within aerosolized particles,” and it seeks research to fill this and other “data gaps.” Other agencies of government should follow this lead.
None of these recommendations speaks directly to the ultimate cause of the problem discussed in this article — the failure of the CDC and the WHO to respond in a timely manner to the concerns voiced by scientists and occupational-safety researchers about the possible transmission of Ebola through the air and how best to guard against it. Fundamentally, this problem is political, rooted in the desire of public health authorities to manage perceptions, to minimize panic, to avoid diplomatic problems, to not make elected officials look bad, and otherwise to give the impression of being on the ball. No simple institutional rearrangement or procedural change will serve as a fix; a political problem requires a political solution. But vigorous oversight from the press and from elected officials can help rebalance priorities and reduce the likelihood that at least this particular problem will recur.
All of this has happened before; all of this need not happen again. In late 2002, an outbreak of SARS spread across the world, with at least eight thousand people infected. The hardest-hit country in the Western Hemisphere was Canada, with the large majority of cases occurring in Ontario. The subsequent investigation of Ontario’s SARS Commission, chaired by the respected Superior Court Justice Archie Campbell, resulted in a scathing final report. It too should be required reading for public health leadership in the United States.
In the Ontario outbreak, nurses and doctors could see that their compatriots were falling ill. Some protested against the weak safety standards — surgical masks, no respirators — but their concerns were stifled and they were assured that science said SARS spread only by droplets.
Again and again, health workers in Ontario were told they were safe if they would only do what they were directed to by the hospitals and the government. Again and again, these confident scientific assurances turned out to be tragically wrong.
Out of hundreds of people infected in Ontario, 45 percent were health care workers, 72 percent contracted the disease in a health care setting, and “in many cases nurses sick with undetected SARS brought illness, and in some cases death, home to their families.”
SARS is now thought to be airborne, in even the conventional sense. A meticulous study of how the disease hit a Hong Kong housing complex during the outbreak found that the virus had in fact spread chiefly through the airborne route, aided by the ventilation system. The CDC currently recommends the use of at least unpowered respirators for workers treating SARS patients.
As with the SARS outbreak, health care workers have been especially vulnerable to Ebola. Given the public health leadership’s aversion to acknowledging the risks, the fact that no health care workers have lost their lives to Ebola in the United States is considerably owed to good fortune. West Africa — where 507 health care workers have died of Ebola, at a fatality rate of 58 percent of infections compared to 40 for their patients — has not been so lucky.
At the height of the Ebola outbreak, Michael Osterholm, the lead author of the mBio paper, offered his colleagues a warning in a remarkable lecture at Johns Hopkins. He cautioned against shrugging at weak data, and failing to acknowledge uncertainty. And, paraphrasing the legendary physicist Richard Feynman, he said, “For a successful public health response, reality must take precedence over public relations, for nature cannot be fooled.” The lecture happened to be delivered on October 20, 2014, the day the CDC at last upgraded its Ebola protection guidelines.
The welter of confusing science and bureaucratic squabbles does not have to be sorted out and adjudicated to appreciate two very basic principles. First: err on the side of caution. As the Ontario commission concluded, “reasonable efforts to reduce risk need not await scientific proof.” Second: tell the truth. Seek what you don’t know, and admit it. The truth, the whole truth, is a greater ally than half-truth against danger. So, too, in the long run if not always the short, against fear.
Physics and respiratory biology. As the body of this article explains, the best understanding of how infectious particles are generated, move through the air, and then are reabsorbed in human tissues offers no reason to believe that the Ebola virus cannot be transmitted through the air. The viruses themselves, as the February 2015 mBio article “Transmission of Ebola Viruses” puts it, are “in the respirable range” of particle sizes.
Persistence in the environment. The limited available evidence suggests that Ebola is less able to remain viable in the environment than diseases like tuberculosis, but still has hardiness comparable to influenza. A 2010 study on the family of viruses to which Ebola belongs suggested that aerosols containing the virus “are able to survive and remain infectious for cell culture” for 104 minutes. The study also found Ebola “could be recovered from contaminated substrates” — meaning surfaces including glass and plastic — “for at least 50 days.” This duration was at temperatures a little above the freezing point of water; the virus generally decayed more quickly at room temperature, with the bulk of persistence falling off within the first seven days. (For comparison, the persistence time on dry surfaces and in dust particles for the smallpox virus is up to a year, while influenza lasts a few days.) However, as noted in a December 2014 literature review published in Environmental Science and Technology Letters, Ebola persistence research is quite limited.
Infectivity. Though Ebola is rather weak at persisting, it is relatively strong in its infective capacity once viable viruses challenge wet tissue. A 1995 study in rhesus monkeys found that four hundred viable particles disseminated in aerosols were sufficient to cause infection. By comparison to non-aerosol transmission, a 1998 study in mice found that a single Ebola particle injected into the abdomen was sufficient to cause infection and kill half of the mice.
Animal models. This is the area of the evidence that received the most meaningful attention from the news media during the outbreak. It is also the most fragmentary and controversial.
The possibility of direct aerosol infection with Ebola has been more or less conclusively demonstrated in nonhuman primates. In the 1995 study mentioned above, which bears the ominous title “Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus,” four monkeys were made to inhale droplets measuring only 0.8–1.2 micrometers and containing the Ebola virus. All were infected and developed hemorrhagic fever. The researchers conducting this experiment in a U.S. Army facility in Frederick, Maryland wore full-body positive-pressure suits.
Another study in 2008, intended to test a potential vaccine, directly exposed macaque monkeys to aerosolized Ebola virus, and found similar results: All three vaccinated macaques escaped infection, while all three unvaccinated macaques developed infection.
These studies do not directly examine transmission between animals, however. In perhaps the most-discussed such study, published in 2012 in Scientific Reports, researchers infected six piglets with Ebola virus through an aerosol mist, and subsequently placed four healthy macaques in individual cages into the pig pen, with a twenty-centimeter barrier between them and the pigs. All four macaques became infected, developed Ebola symptoms, and were euthanized. The authors concluded that the finding “supports the concept of airborne transmission” of Ebola. However, the study did not allow for determination of what size of droplets might have been involved, nor did it eliminate the possibility of droplets created and spread during cage cleaning.
A 1995 study published in The Lancet placed three uninfected monkeys in cages ten feet away from infected ones. Two became infected with Ebola. The researchers describe stringent decontamination practices that made droplet or surface transmission unlikely, and they concluded, “exposure to airborne droplets of the virus had to be considered as the most likely mode of infection.” A 2014 study, however, conducted during the outbreak, placed healthy macaques in open-barred cages one foot away from Ebola-infected macaques, and no infection occurred, either via airborne particles, droplets, or any other mode of transmission.
This research and its significance remain hotly contested. Critics point to methodological limitations and flaws, while opinion remains divided on whether human physiology is sufficiently similar to these animals for the studies to be relevant. Even so, although it is far from clear that this data confirms the possibility of Ebola transmission through air between humans, it is still less clear that the possibility can be ruled out.
Epidemiology. In some prior outbreaks, rigorous contact tracing has shown some portion of cases that could not be attributed to a patient having had direct physical contact with another infected person. In some cases not even close proximity to an infected patient could be shown.
In a study of one of the first outbreaks, in 1976 in Nzara, Sudan, thorough contact tracing found that “no contact with a previous case could be established” for 21 percent of patients. In a study of the 1995 outbreak in DR Congo, out of 316 patients traced, 55 had no initially reported exposure source. A subsequent investigation of 44 of these patients found that 19 had no exposure other than visiting but not providing medical care to an Ebola patient. Five of these were found not to have touched the patient. An additional 12 patients had not been in proximity to an infected person at all, nor been admitted to a hospital or experienced any other known risk factors.
This research was conducted under difficult circumstances, and in many cases answers came from relatives of patients who had died. Consequently, critics have argued that these results may simply be incomplete. Nonetheless, if this data does not conclusively demonstrate Ebola transmission through air, it also does not rule it out. Moreover, as Lisa Brosseau and Rachael Jones have argued, establishing that a patient had previously cared for or touched another infected patient does not in itself demonstrate what was the mechanism of transmission, and fine aerosol transmission is likeliest at the same close range where droplet and contact transmission occur.
Field history of respiratory protective equipment. Perhaps the most compelling evidence of Ebola transmission through the air is this: Health care workers who do not wear respirators often get infected with Ebola, while those who do wear them almost never get infected.
In the most recent outbreak, in the United States, we have seen the cases of the two infected nurses in Dallas, who were not wearing respirators. Atlanta’s Emory University Hospital, which required respirators, successfully treated four Ebola patients (including one of the Dallas nurses), with no infections of health care workers.
In West Africa, Doctors Without Borders treatment centers enforced stringent protections, including full-body hazmat suits and respirators. At the end of October 2014, they reported that no more than 23 of their over 3,300 staff members working on Ebola in West Africa had contracted the disease. Investigations found that two of these infections were “due to chance encounters in a triage area,” while the “vast majority” occurred outside of the treatment centers, when native workers returned to their communities. The one unexplained case is Craig Spencer, the American doctor who became ill after returning to New York.
Outside Doctors Without Borders facilities, in the region at large, many hospitals did not even observe droplet precautions. Those that did rarely had workers wearing respiratory protection stronger than surgical masks. In the current outbreak, West Africa has seen 869 health care workers infected with Ebola as of the end of June 2015.
October 20, 2015
During Covid, The New Atlantis has offered an independent alternative. In this unsettled moment, we need your help to continue.